➔ Calculate True Specific Gravity
➔ Calculate Bulk Specific Gravity
➔ Calculate Apparent Solid Specific Gravity
➔ Calculate Apparent Porosity
➔ Calculate Water Absorption
➔ Calculate True Porosity
Properties of Porous Solids
Porous solids are materials that contain a significant amount of void or empty space within their structure, making them distinct from solid, dense materials. These voids or pores give porous solids unique properties, which can make them highly suitable for various applications such as catalysis, filtration, storage, and insulation. The properties of porous solids are influenced by their pore structure, size distribution, and material composition.
Key Properties of Porous Solids:
-
Porosity:
- Porosity is the measure of void spaces in a material and is expressed as the ratio of the volume of voids (pores) to the total volume of the material.
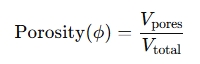
- Porosity ranges from micro (less than 2 nm), meso (2-50 nm), to macro pores (greater than 50 nm).
- Materials with high porosity typically have large surface areas, which makes them useful in applications like adsorption and catalysis.
-
Specific Surface Area:
- The specific surface area of porous solids refers to the surface area per unit mass or volume and is a key factor in determining their effectiveness in applications such as adsorption and catalysis.
- It is commonly measured in m²/g (square meters per gram) and can be determined using techniques like BET (Brunauer–Emmett–Teller) analysis, which involves nitrogen adsorption.
- A high specific surface area allows porous solids to store more molecules or participate in more reactions.
-
Pore Size Distribution:
- Pore size distribution is a critical property that influences how a material interacts with molecules, liquids, or gases.
- Micropores (pore sizes less than 2 nm): Common in materials like activated carbon or zeolites. These are suitable for small molecule adsorption.
- Mesopores (pore sizes between 2 nm and 50 nm): Found in materials like silica gels and alumina. These pores are good for storage and filtering applications.
- Macropores (pore sizes greater than 50 nm): Often found in materials like sponges or certain types of ceramic foams, which are used for larger scale flow applications.
- Pore size distribution is a critical property that influences how a material interacts with molecules, liquids, or gases.
-
Permeability:
- Permeability refers to the ability of a porous material to allow fluids or gases to pass through it. It is influenced by both the size and connectivity of pores.
- High permeability is desirable in applications such as filters, membranes, or catalysts, where fluid or gas flow through the material is required.
-
Mechanical Strength:
- The mechanical strength of porous solids typically decreases as their porosity increases. Highly porous materials tend to be brittle and may fracture under stress.
- The strength can be improved through the choice of material or by modifying the pore structure (e.g., by creating a more ordered or rigid pore network).
- Some porous materials, like aerogels or foams, have a low density and are mechanically lightweight, while still maintaining useful strength for certain applications.
-
Thermal Conductivity:
- Porous materials generally have lower thermal conductivity than solid materials due to the air or gas trapped in the pores, which acts as an insulator.
- This property makes porous solids useful in thermal insulation (e.g., ceramic foams, aerogels, and thermal insulators in building materials).
- The pore size and porosity will affect the thermal conductivity; smaller pores or high porosity usually result in better insulation.
-
Adsorption Capacity:
- Porous solids, especially those with micropores and mesopores, are highly effective at adsorbing molecules from gases or liquids.
- This property is useful in applications like gas storage, separation processes, water filtration, and catalysis.
- The adsorption isotherms (e.g., Langmuir or Freundlich models) are often used to characterize the adsorption behavior of porous materials.
-
Capillarity:
- Capillarity or capillary action refers to the ability of a porous material to draw fluids into its pores through surface tension. This is a significant property in materials like soil, porous ceramics, and membranes.
- This phenomenon can affect how fluids are transported through porous media, especially in hydrophobic or hydrophilic materials.
-
Chemical Resistance:
- Many porous materials are chemically resistant and can be used in aggressive environments (e.g., in catalysis or adsorption for chemical processes).
- The chemical stability of the material depends on its composition, such as silica, alumina, or carbon-based materials, which are resistant to many acids, bases, and solvents.
-
Electrical Properties:
- Some porous materials, such as porous carbons or conductive polymers, can have conductive properties and are used in electrochemical applications (e.g., batteries or supercapacitors).
- The conductivity of these materials depends on the size of the pores, electrical connectivity, and material composition.
Types of Porous Solids:
-
Porous Ceramics:
- Porous ceramics are widely used in filtration, insulation, and structural applications. These can include alumina, silica, and zirconia.
- Examples include ceramic foams, filter media, and catalysts for chemical reactions.
-
Activated Carbon:
- Activated carbon has a very high surface area and is commonly used for adsorption of gases, liquids, and impurities.
- It is often used in water purification, air filtration, and gas masks.
-
Aerogels:
- Aerogels are lightweight, highly porous materials with extremely low thermal conductivity. They are used in thermal insulation, catalysis, and space missions.
- Silica aerogels are some of the most common types used for high-performance insulation.
-
Polymeric Foams:
- Polymeric foams, such as polyurethane and polystyrene, are used in packaging, insulation, and cushioning.
- These materials have good mechanical properties and are lightweight while maintaining some level of flexibility.
-
Zeolites:
- Zeolites are a class of microporous minerals used extensively in catalysis, adsorption, and ion-exchange processes.
- Their pore structure and high surface area make them ideal for applications like gas separation and water purification.
-
Metal-Organic Frameworks (MOFs):
- MOFs are materials composed of metal ions connected by organic ligands, creating a porous structure with extremely high surface areas.
- They are increasingly used in gas storage, catalysis, and drug delivery systems due to their customizable pore structures.
-
Porous Metals:
- Porous metals are used in applications where a combination of high strength and porosity is needed, such as in lightweight structural components, filters, and heat exchangers.
- These materials combine metallic strength with high porosity and can have tailored pore sizes for specific applications.
Applications of Porous Solids:
-
Catalysis:
- Porous solids are essential in catalysis because their large surface area allows for greater interaction between reactants and catalyst sites, increasing reaction rates.
-
Filtration:
- Porous materials are commonly used for filtering gases and liquids, such as in water treatment plants, air purification systems, and fuel filters.
-
Energy Storage:
- Porous carbons and MOFs are used in applications like batteries and supercapacitors due to their high surface area and ability to store ions.
-
Insulation:
- Aerogels and porous ceramics are used in highly efficient thermal insulation, especially in industries like aerospace, construction, and automotive.
-
Drug Delivery:
- Porous solids like MOFs and silica nanoparticles are increasingly used for controlled drug release, where the drugs are adsorbed into the porous structure and released over time.
-
Structural Components:
- Porous solids like porous metals and ceramic foams are used in applications where lightweight materials are needed without compromising structural integrity, such as in aerospace and automotive industries.