Charles' Law Calculator
What is Charles' Law?
Charles' Law (also known as the law of volumes) describes how gases expand when heated at constant pressure. It states that the volume of a gas is directly proportional to its temperature (in Kelvin) when pressure and the amount of gas are kept constant.
The equation is:
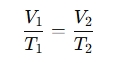
Where:
V₁ = Initial volume of the gas
T₁ = Initial temperature (in Kelvin)
V₂ = Final volume of the gas
T₂ = Final temperature (in Kelvin)
Why it's important:
Charles' Law is crucial for understanding how gases behave when subjected to changes in temperature, like in hot air balloons, engines, and climate science. It shows that heating a gas causes it to expand, which is important in predicting the behavior of gases in real-world applications.
How it works:
Increase in temperature: If you heat a gas at constant pressure, its molecules gain energy, which causes them to move faster. As a result, the gas expands, increasing its volume.
Decrease in temperature: Lowering the temperature reduces the kinetic energy of the molecules, causing the gas to contract and reduce its volume.
When it's used:
Charles' Law is applied when:
Pressure is constant: This law works only if the gas is free to expand or contract, meaning pressure doesn't change.
Temperature and volume are measured: It's used in situations where you need to predict the volume of gas at different temperatures, like in hot air ballooning, weather balloons, and internal combustion engines.
Charles' Law Calculator:
A Charles' Law Calculator helps you quickly compute the volume or temperature of a gas when the other variables are changed, using the relationship from the equation:
Input: You would input the initial and final temperatures (in Kelvin) and the initial volume.
Output: The calculator will give you the new volume (or temperature) of the gas.
It's particularly useful for making predictions in scenarios where the temperature of a gas changes but the pressure remains constant.