Dilution Of Solutions Calculator
What is a Dilution of Solutions Calculator?
A dilution of solutions calculator is a tool that helps determine how to dilute a concentrated solution to achieve a desired concentration and volume. It’s based on the principle of the dilution equation:
Where:
- C1 = Initial concentration (stock solution)
- V1 = Volume of stock solution needed
- C2 = Final concentration (diluted solution)
- V2 = Final volume of the diluted solution
Why use a Dilution of Solutions Calculator?
- Saves time: Quickly calculates the precise amount of stock solution and solvent required.
- Avoids errors: Reduces the risk of miscalculations, especially in labs or industrial processes.
- Standardizes solutions: Ensures consistency when preparing chemical or pharmaceutical solutions.
- Practical for teaching: Helps students learn and verify their dilution calculations.
How does it work?
- Input the initial concentration () — the strength of the stock solution.
- Input the final concentration ( ) — the desired strength after dilution.
- Input the final volume ( ) — the total volume of the diluted solution you need.
- Calculate the volume of the stock solution ( ) — the amount of concentrated solution required.
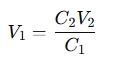
- Calculate the volume of solvent needed:
Solvent volume=V2−V1
When do you use it?
- In chemical labs for preparing reagents.
- In medicine for diluting drugs or solutions.
- In cooking for adjusting the strength of flavoring solutions.
- In industrial processes like wastewater treatment or manufacturing.