Gay-Lussac's Law Calculator
What is Gay-Lussac's Law?
Gay-Lussac's Law describes the relationship between the pressure and temperature of a gas when the volume is held constant. It states that the pressure of a gas is directly proportional to its absolute temperature (in Kelvin) if the volume is constant.
The equation is:
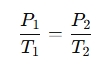
Where:
- P₁ = Initial pressure
- T₁ = Initial temperature (in Kelvin)
- P₂ = Final pressure
- T₂ = Final temperature (in Kelvin)
Why it's important:
Gay-Lussac's Law is important because it shows how pressure and temperature are related in a closed system with constant volume. It is widely used in gas storage, engine technology, and safety calculations where temperature and pressure change but volume is fixed (like in gas cylinders or pressure cookers).
It also helps explain how heating a gas increases its pressure when the volume is confined (think of a pressurized can or aerosol).
How it works:
- Increasing the temperature: If you increase the temperature of a gas in a fixed volume, the gas particles move faster, causing more frequent collisions with the walls of the container. This increases the pressure.
- Decreasing the temperature: If the temperature decreases, the particles move slower, leading to less frequent collisions and a decrease in pressure.
So, when temperature rises, pressure increases, and when temperature drops, pressure decreases—provided the volume remains constant.
When it's used:
Gay-Lussac's Law is particularly useful when:
- Volume is constant: The law applies when gas is in a rigid container that doesn't allow expansion or compression (e.g., gas cylinders, sealed pressure vessels).
- Pressure and temperature change: You need to predict the pressure of a gas after a temperature change or vice versa, without knowing the volume.
Gay-Lussac's Law Calculator:
A Gay-Lussac's Law Calculator allows you to calculate the pressure of a gas (or the temperature) when the temperature (or pressure) changes, with the assumption that volume is constant.
- Input: You'd input the initial pressure, initial temperature, and either the final temperature or pressure.
- Output: The calculator would give you the missing value (final pressure or temperature).
For example:
- If you know the initial pressure of a gas and its temperature, you can calculate how the pressure will change if the temperature changes, provided the volume stays constant.
This is used in:
- Pressurized containers: For understanding how temperature changes affect the pressure of gases in sealed containers.
- Weather balloons: To estimate how the pressure changes with the temperature as the balloon rises.
- Engine calculations: In combustion engines or air conditioning systems, where gases are heated or cooled in a confined space.
In short, the calculator helps quickly compute the effects of temperature changes on pressure when volume is fixed.